

Valine Side Effects: BCAA Health Risks & Benefits
By Steve Blechman
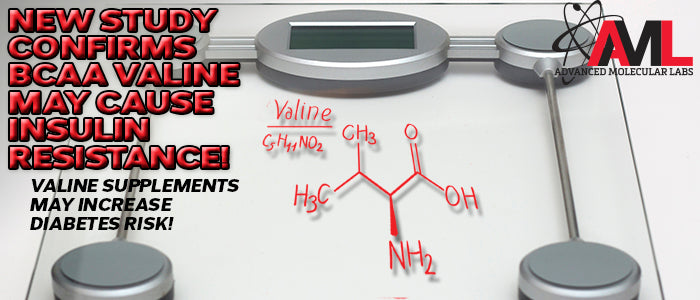
Branched-chain amino acids supplements (BCAAs) have been associated with insulin resistance in obese individuals. Recently, the valine catabolite 3-hydroxyisobutyrate (3-HIB) was shown to promote insulin resistance in skeletal muscle by increasing lipid content in vivo. The purpose of this study was to investigate the mechanistic effects of 3-HIB on skeletal muscle insulin signaling, metabolism, and related gene expression in vitro. These findings were recently published in the Journal of Nutrition Research (June 2019). This most recent study found that 3-HIB can reduce muscle insulin sensitivity and support a role of 3-HIB in the development of insulin resistance. This study is significant because it confirms the mechanism of 3-HIB and insulin resistance in muscle, highlighting potential valine side effects!
Research has shown that elevated levels of valine are present in the blood of diabetic rats, mice and humans. (Nat Rev Endocrinol, 2014). When the mice were fed a diet without valine, insulin sensitivity improved after only one day. Mice on the valine-free diet lasting an entire week decreased their blood glucose levels, indicating that there was improved insulin function. (Metabolism, 2014). It was reported in the journal Nature Medicine in 2015 that valine catabolite 3-hydroxyisobutyrate (3-HIB) promoted the accumulation of fat within muscle tissue by directly stimulating fatty uptake in the muscle. The intramuscular fat activates certain signaling cascades within the muscle cell that diminish insulin signaling, leading to insulin resistance. This study also found that inhibiting the production of 3-HIB prevented the uptake of fat. Other studies support the negative effect of 3-HIB on insulin signaling with elevated 3-HIB in the muscle of human subjects with diabetes. (J Lipid Res, 1989; Diabetologia, 2015). These findings suggest that bcaa health risks, particularly those related to bcaa valine, should be carefully considered.
More recent studies have confirmed that the branched-chain amino acid valine metabolite 3-HIB is involved in the pathogenesis of insulin resistance in skeletal muscle and might be involved in insulin resistance in humans (Diabetes, July 2017; EbioMedicine, 2018; J Diab Rsch, 2018). Unlike valine, leucine has been shown to improve insulin function. Leucine consumption alone has been shown to rescue insulin-signaling deficiency (PLoS, 2011). A most recent study (Exp Clin Endocrinol Diabetes, 2018) found that oral administration of leucine improved endothelial function in healthy individuals when infused with glucose. Acute hyperglycemia impairs endothelial function in healthy individuals. This study found that leucine administration prevented hyperglycemia-mediated endothelial function. Unlike leucine, which avoids insulin resistance by increasing mitochondrial-driven fat loss, valine does not encourage mitochondrial biogenesis. Impaired mitochondrial function in skeletal muscle is one of the major predisposing factors to metabolic diseases, such as insulin resistance, type 2 diabetes and cardiovascular disease. Leucine supplementation increases insulin sensitivity by activating SIRT1 activity. SIRT1 is known to "promote mitochondrial biogenesis and oxidative capacity and prevent the mitochondrial dysfunction in skeletal muscle" (Journal of Nutrition and Metabolism, 2014). Leucine may also attenuate adiposity and promote weight loss during energy restriction (Nutrition 2006, Diabetes, 2007). These effects are in part by activating the SIRT1-dependent pathway, stimulating mitochondrial biogenesis and increased oxygen consumption (Nutrition Metabolism, 2008). Mitochondrial biogenesis and SIRT1 expression in skeletal muscle has also been shown to increase life span in middle-aged mice (Cell Metabolism, 2010). As far as isoleucine is concerned, unlike valine, it has been shown to improve insulin sensitivity by increasing glucose into muscle cells (Am J Physiol Endocrinol Metab, 2007). The differences in leucine isoleucine and valine side effects highlight the importance of understanding individual amino acid impacts.
It's clear based on scientific research that the high-circulating BCAA valine is associated with obesity and diabetes. The latest available literature has shown that the branched-chain amino acid valine (catabolite 3-HIB) is most likely the probable cause of insulin resistance, raising concerns about bcaa supplements and diabetes!
References:
Lyon, Emily S; Rivera, Madison E; Johnson, Michele A; Sunderland, Kyle L; Vaughan, Roger A. Actions of chronic physiological 3-hydroxyisobuterate treatment on mitochondrial metabolism and insulin signaling in myotubes. Nutrition Research, June 2019. Vol: 66, Page: 22. DOI 10.1016/j.nutres.2019.03.012
Cummings, NE, Williams, EM, Kasza, I, Konon, et al. (2018), Restoration of metabolic health by decreased consumption of branched‐chain amino acids. J Physiol, 596: 623-645. doi:10.1113/JP275075
Duke University. "Diabetes researchers find switch for fatty liver disease: Carbs, fats and protein: One molecule to rule them all?" ScienceDaily, 17 May 2018. www.sciencedaily.com/releases/2018/05/180517113847.htm
PJ White, RW McGarrah, PA Grimsrud, S Tso, W Yang, JM Haldeman, C Newgard et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metabolism, 2018; DOI: 10.1016/j.cmet.2018.04.015
NutraIngredients-USA.com, December 17, 2017. Nathan Gray. Could dropping specific amino acids from diet be key to weight loss?
CB Newgard, J An, JR Bain et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance Cell Metab, 2009 April; 9(4): 311-326. doi:10.1016/j.cmet.2009.02.002
Zheng Y, Li Y, Qi Q et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol, 2016; 45: 1482-92.
Isanejad M, LaCroix AZ, Thomson CA et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women's Health Initiative. Br J Nutr, 2017; 117:1523-30.
Newgard CB, An J, Bain JR et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab, 2009; 9: 311-26
Tremblay F, Krebs M, Dombrowski L et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes, 2005; 54: 2674-84.
Tremblay F, Krebs M, Dombrowski Let al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes, 2005; 54: 2674-84.
Lee CC, Watkins SM, Lorenzo C et al. Branched-chain amino acids and insulin metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care, 2016; 39: 582-8.
Yoon M-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Forum Nutr, 2016; 8: 405-17.
Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care, 2006; 29:1130-9.
Binder E, Bermudez-Silva FJ, Andre C et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS One, 2013; 8:e74705.
Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes, 2007; 56: 1647-54.
Lynch, C.J. and Adams, SH (2014) Branched-chain amino acids in metabolic signaling and insulin resistance. Nat Rev Endrocrinol 10, 723-736.
Xiao, F, Yu, J, Guo, Y, Deng, J, Li, K, et al (2014) Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 63, 841-850.
Jang C, Oh, SF, et al A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22, 421-426
Sara B Seidelmann, Brian Claggett, Susan Cheng, Mir Henglin, Amil Shah, Lyn M Steffen, Aaron R Folsom, Eric B Rimm, Walter C Willett, Scott D Solomon, Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis, The Lancet Public Health, 2018, ISSN 2468-2667, https://doi.org/10.1016/S2468-2667(18)30135-X.
Zhaoping Li, Professor of Medicine, UCLA, US National Library of Medicine www. ClinicalTrials.gov Identifier: NCT02684565 Last Update Posted: February 12, 2018 https://clinicaltrials.gov/ct2/show/NCT02684565
Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, et al. (2016) Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLOS Medicine 13(11): e1002179. https://doi.org/10.1371/journal.pmed.1002179
Avogaro, A and Bier, DM (1989.) Contribution of 3-hydroxyisobutyrate to the measurement of 3-hydroxybutyrate in human plasma: comparison of enzymatic and gas-liquid chromatography-mass spectrometry assays in normal and in diabetic subjects. J Lipid Res 30, 1811-1817
Giesbertz, P, Padberg, I, et al. (2015) Metabolite profiling in plasma and tissues of ob/ob and db/db mice identifies novel markers of obesity and type 2 diabetes. Diabetologia 58, 2133-2143.
Insulin Resistance, And What May Contribute To It by Lila Abassi on March 14, 2016. American Council on Science & Health https://www.acsh.org/news/2016/03/14/branched-chain-amino-metabolite-a-culprit-in-insulin-resistance
Alterations in 3-Hydroxyisobutyrate and FGF21 Metabolism Are Associated With Protein Ingestion-Induced Insulin Resistance Lydia-Ann LS Harris, Gordon I Smith, Bruce W Patterson, Raja S Ramaswamy et al. Diabetes 2017;66:1871-1878 https://doi.org/10.2337/db16-1475
Elevated Plasma Levels of 3-Hydroxyisobutyric Acid Are Associated With Incident Type 2 Diabetes Mardinoglu, Adil et al. EBioMedicine, Volume 27, 151-155, Jan. 2018.
Ulrika Andersson-Hall, Carolina Gustavsson, Anders Pedersen, Daniel Malmodin, Louise Joelsson, and Agneta Holmäng, "Higher Concentrations of BCAAs and 3-HIB Are Associated with Insulin Resistance in the Transition from Gestational Diabetes to Type 2 Diabetes," Journal of Diabetes Research, vol. 2018, Article ID 4207067, 12 pages, 2018. https://doi.org/10.1155/2018/4207067.
Macotela, Y Emanuelli, B, Bang, AM et al. (2011) Dietary leucine - an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 6, e21187.
Branched Chain Amino Acids: Beyond Nutrition Metabolism. Cunxi Nie, Ting He, Wenju Zhang, Guolong Zhang, Xi Ma Int J Mol Sci. 2018 Apr; 19(4): 954. Published online 2018 Mar 23. doi: 10.3390/ijms19040954
Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Y. Zhang, K. Guo, R.E. LeBlanc, D. Loh, G.J. Schwartz, Y. Yu Diabetes Jun 2007, 56 (6) 1647-1654; DOI: 10.2337/db07-0123
Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus Deirdre K Tobias, Clary Clish, Samia Mora, Jun Li, Liming Liang, Frank B Hu, JoAnn E. Manson, Cuilin Zhang Clinical Chemistry Aug 2018, 64 (8) 1203-1210; DOI: 10.1373/clinchem.2017.285841
McCormack, SE, Shaham, O, McCarthy, MA, Deik, AA, Wang, TJ, Gerszten, RE, Clish, CB, Mootha, VK, Grinspoon, SK and Fleischman, A. (2013), Branched‐chain amino acids and IR in children. Pediatric Obesity, 8: 52-61. doi:10.1111/j.2047-6310.2012.00087.x.
Sina S Ullrich, Penelope CE Fitzgerald, Gudrun Schober, Robert E Steinert, Michael Horowitz, Christine Feinle-Bisset; Intragastric administration of leucine or isoleucine lowers the blood glucose response to a mixed-nutrient drink by different mechanisms in healthy, lean volunteers, The American Journal of Clinical Nutrition, Volume 104, Issue 5, 1 November 2016, Pages 1274-1284, https://doi.org/10.3945/ajcn.116.140640.
Branched-chain and aromatic amino acids, insulin resistance and liver specific ectopic fat storage in overweight to obese subjects. Haufe, S. et al. Nutrition, Metabolism and Cardiovascular Diseases , Volume 26 , Issue 7, 637-642
Li, H, Xu, M, Lee, J, He, C, & Xie, Z (2012). Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. American Journal of Physiology - Endocrinology and Metabolism, 303(10), E1234-E1244. http://doi.org/10.1152/ajpendo.00198.2012
Chunzi Liang, Benjamin J Curry, Patricia L Brown, and Michael B Zemel, "Leucine Modulates Mitochondrial Biogenesis and SIRT1-AMPK Signaling in C2C12 Myotubes," Journal of Nutrition and Metabolism, vol. 2014, Article ID 239750, 11 pages, 2014. https://doi.org/10.1155/2014/239750.
Leucine Supplementation Protects from Insulin Resistance by Regulating Adiposity Levels. Binder E, Bermúdez-Silva FJ, André C, Elie M, Romero-Zerbo SY, et al. (2013) Leucine Supplementation Protects from Insulin Resistance by Regulating Adiposity Levels. PLOS ONE 8(9): e74705. https://doi.org/10.1371/journal.pone.0074705
Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Banerjee, Jheelam et al. Metabolism - Clinical and Experimental, Volume 65 , Issue 11 , 1679-1691
Jiao, J, Han, S-F, Zhang, W, Xu, J-Y, Tong, X, Yin, X-B, Qin, L-Q (2016). Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food & Nutrition Research, 60, 10.3402/fnr.v60.31304. http://doi.org/10.3402/fnr.v60.31304
Published: 07 March 2016. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Cholsoon Jang, Sungwhan F Oh, Shogo Wada, Glenn C Rowe, Laura Liu, Mun Chun Chan, James Rhee, Atsushi Hoshino, Boa Kim, Ayon Ibrahim, Luisa G Baca, Esl Kim, Chandra C Ghosh, Samir M Parikh, Aihua Jiang, Qingwei Chu, Daniel E Forman, Stewart H Lecker, Saikumari Krishnaiah, Joshua D Rabinowitz, Aalim M Weljie, Joseph A Baur, Dennis L Kasper & Zoltan Arany Nature Medicine volume 22, pages 421-426 (2016)
D'Antona, G, Ragni, M, Cardile, A, Tedesco, L, Dossena, M, Bruttini, F et al. (2010). Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 12, 362-372. doi: 10.1016/j.cmet.2010.08.016
Szmelcman S, Guggenheim K. Interference between leucine, isoleucine and valine during intestinal absorption. Biochemical Journal. 1966;100(1):7-11.
Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? Robert R Wolfe Journal of the International Society of Sports Nutrition201714:30 https://doi.org/10.1186/s12970-017-0184-9
Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids, 2008, Volume 35, Number 1, Page 147 L. Deldicque, C. Sanchez Canedo, S. Horman,
Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. Amy Hector, Stuart M. Phillips Int J Sport Nutr Exerc Metab. 2017
Leucine, Not Total Protein, Content of a Supplement Is the Primary Determinant of Muscle Protein Anabolic Responses in Healthy Older Women, The Journal of Nutrition, nxy091, June 13, 2018
Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Rahimi MH, Shab-Bidar S, Mollahosseini M, Djafarian K. Nutrition. 2017





